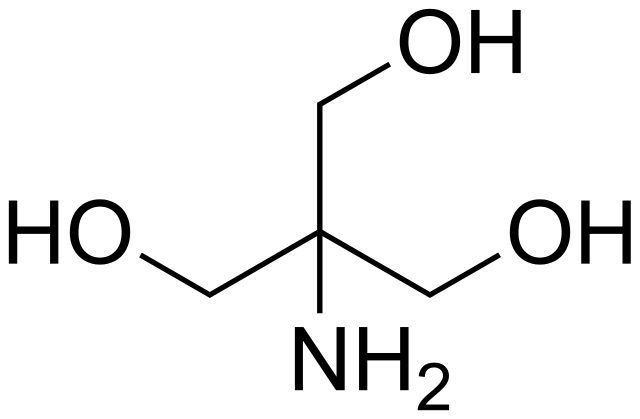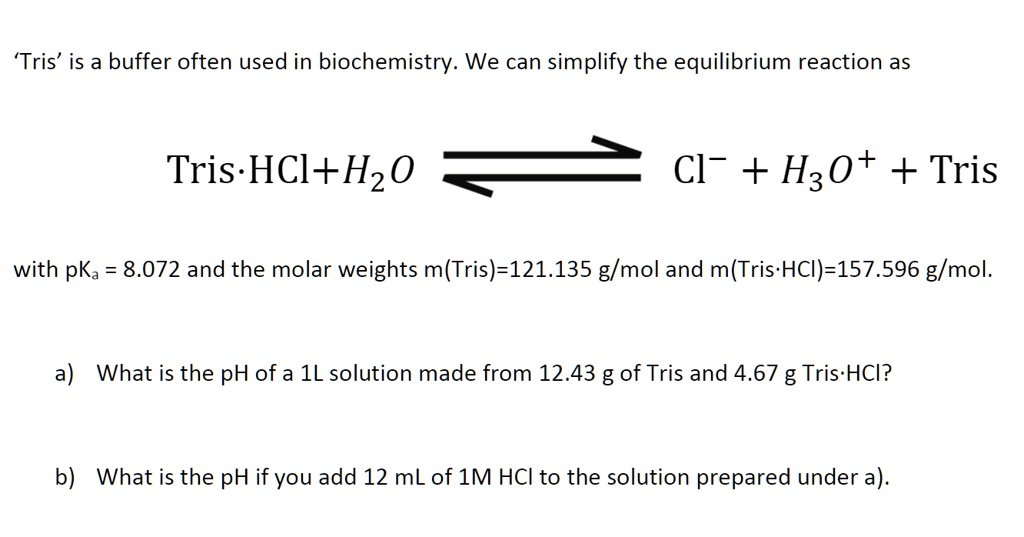
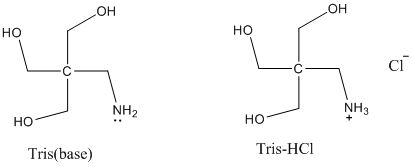
SOLVED: Tris' is a buffer often used in biochemistry. We can simplify the equilibrium reaction as: Tris-HCl + H2O -> Cl- + H3O+ + Tris with pKa = 8.072 and the molar

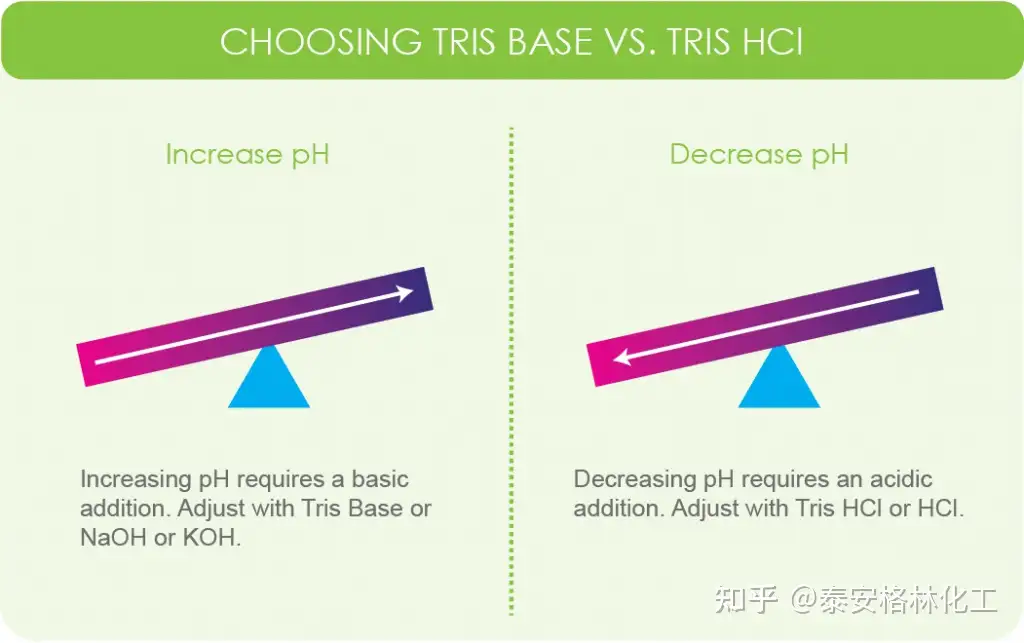
SOLVED: Question 2 (1 point) A buffer is made using only Tris (weak base) and Tris HCl (weak acid): What mass (in g) of solid Tris HCl would be used to make

Interaction of Tris with DNA molecules and carboxylic groups on self-assembled monolayers of alkanethiols measured with surface plasmon resonance - ScienceDirect
Solved: Chapter 17 Problem 92AP Solution | Loose Leaf Version For Chemistry: Atoms First 2nd Edition | Chegg.com

New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions - ScienceDirect
TRIS hydrochloride, 50 g, CAS No. 1185-53-1 | Reagents for protein isolation | Protein Isolation | Biochemistry | Life Science | Carl Roth - International


![T60050-500.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 500 Grams T60050-500.0 - TRIS Hydrochloride [Tris(hydroxymethyl) aminomethane HCl], 500 Grams](https://d2gdaxkudte5p.cloudfront.net/system/images/T60050-500.0.jpg)