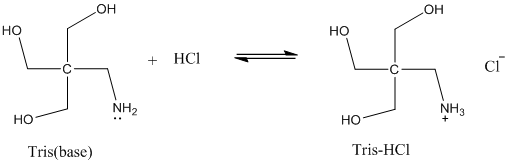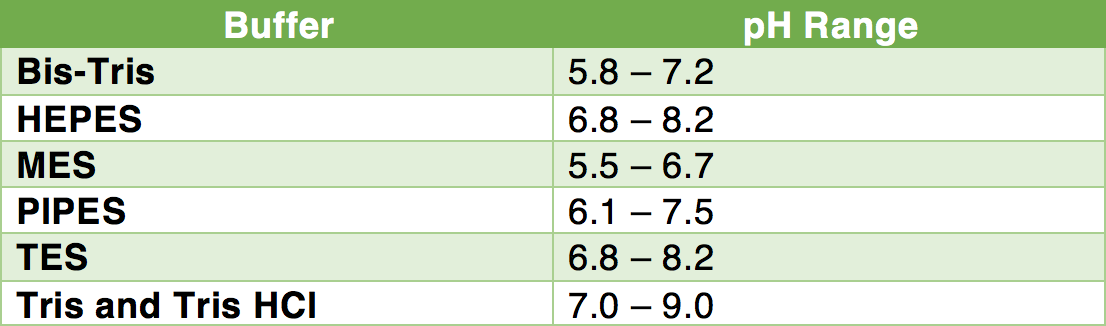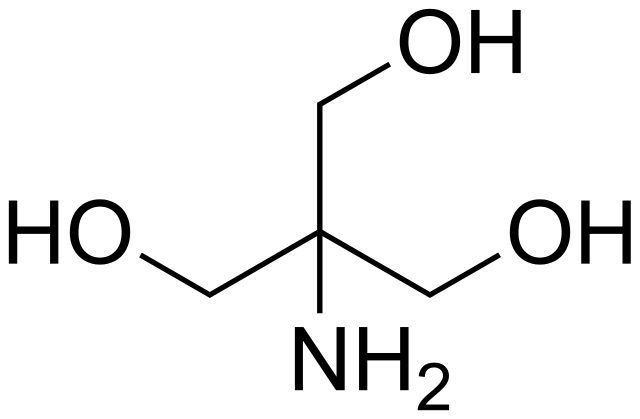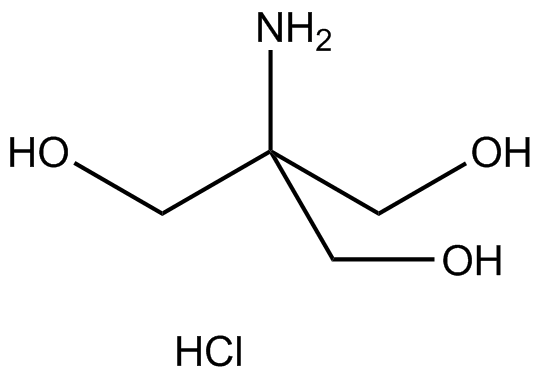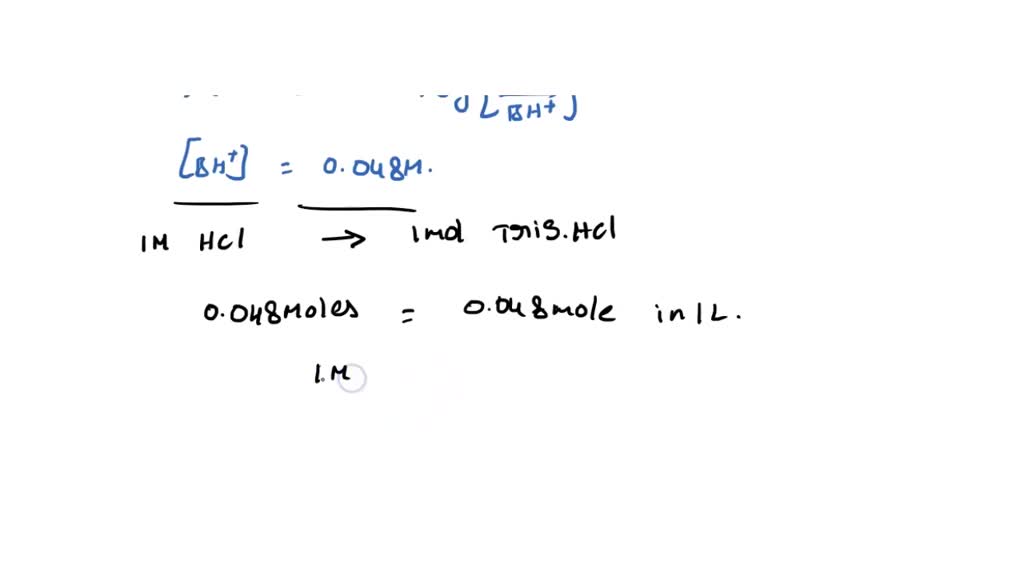
SOLVED: Calculate how you world prepare 100 mL of a 0.1 M TRIS-HCl buffer, pH 7.4 using solid TRIS base (tris-(hydroxymethyl)-aminomethane) (MW 121.1, pKa 8.08). You may find TRIS under several different

Interaction of Tris with DNA molecules and carboxylic groups on self-assembled monolayers of alkanethiols measured with surface plasmon resonance - ScienceDirect

Scheme 2. Structures of Tris, TrisHCl and etilendiaminotetraacetic acid (H4EDTA) : pH and Acid-Base Equilibrium Calculations via a Matrix Representation of Solutions of Acids and/or Bases : Science and Education Publishing

New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions - ScienceDirect

Measurement of pHT values of Tris buffers in artificial seawater at varying mole ratios of Tris:Tris·HCl - ScienceDirect

![Tris Base [C4H11NO3] Molecular Weight Calculation - Laboratory Notes Tris Base [C4H11NO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2022/11/tris-base-molecular-weight-calculation-300x204.jpg)


![BT156] 2M Tris-HCl, pH 9.5 | Biosolution BT156] 2M Tris-HCl, pH 9.5 | Biosolution](http://biosolution.cafe24.com/wp-content/uploads/2015/07/BT066-Tris-HCl.jpg)